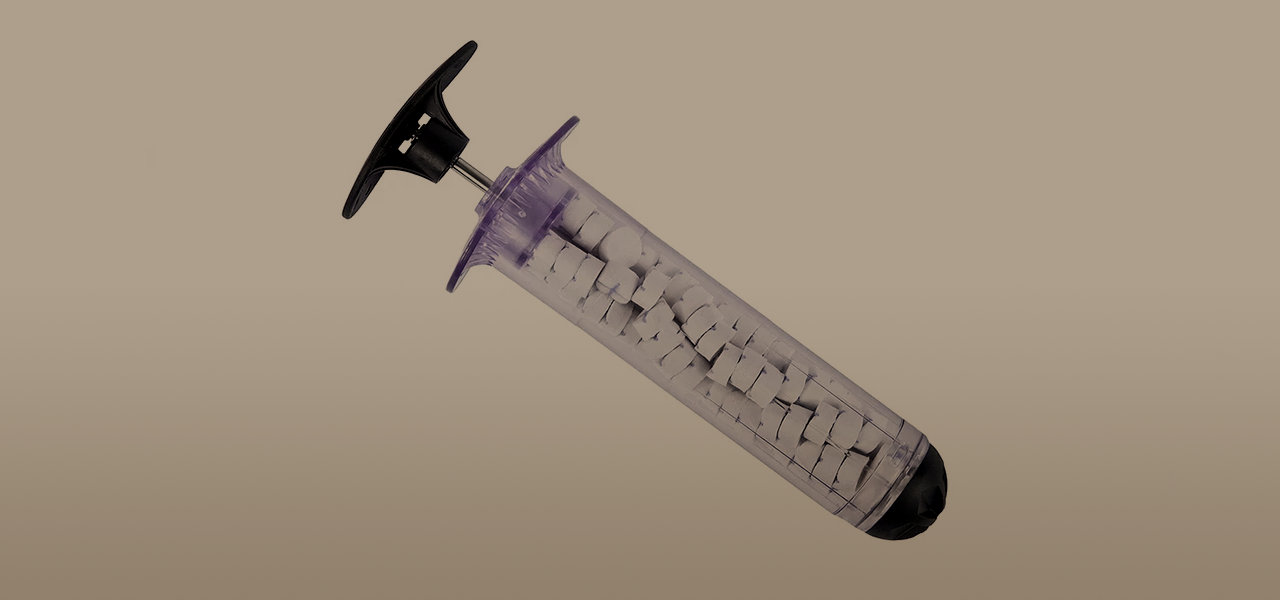
RevMedx, Inc. is a startup company that has developed a portfolio of devices designed specifically for civilian first responders and combat medics that have revolutionized the treatment of traumatic bleeding. Their flagship product, XSTAT®, is a first-in-kind hemostatic device for the control of severe, life-threatening bleeding from junctional wounds in the groin or axilla not amenable to tourniquet application in adults and adolescents.
RevMedx was formed in 2010 by a group of combat veterans, scientists and engineers. It is well known that hemorrhaging or “bleeding out” from wounds on the battlefield is the leading cause of death for soldiers. The RevMedx team developed the new XSTAT product, a device that can be rapidly deployed where it may be impossible to put a tourniquet or apply manual compression externally, to provide fast-acting hemorrhage control to stabilize a wounded patient for transport. The XSTAT product was granted FDA approval in April of 2014. Today RevMedx manufactures the XSTAT product out of a cleanroom facility in Wilsonville, OR. RevMedx has developed a suite of products that work in conjunction with XSTAT and continues to conduct research on new and innovative medical products.